
| N/A |
| Packing | |
|---|---|
| Storage | Powder -20°C 3 years 4°C 2 years;In solvent -80°C 6 months, -20°C 1 month |
| Shipping | Room temperature in continental US; may vary elsewhere |
Tel: 0086-25-52397805
Email: sales7@alchemist-chem.com

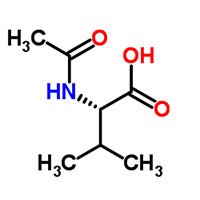
| Common Names | Acetylvaline | ||
|---|---|---|---|
| Structure |  |
||
| CAS No. | 96-81-1 | Boiling Point (℃) | 362.2±25.0 °C at 760 mmHg |
| Molecular Weight | 159.183 | Melting Point (℃) | 165 °C |
| Density | 1.1±0.1 g/cm3 | Vapor Specific Gravity | N/A |
| Molecular Formula | C7H13NO3 | Flash Point (℃) | 172.8±23.2 °C |
| Solubility | N/A | Autoignition Temperature (℃) | N/A |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter | ||
|---|---|---|---|
| Hazard Codes | T+:Very toxic | ||
| Safety Phrases | S1-S28-S45 | ||
| RIDADR | NONH for all modes of transport | ||
| WGK Germany |