
| N/A |
| Packing | |
|---|---|
| Storage | Powder -20°C 3 years 4°C 2 years;In solvent -80°C 6 months, -20°C 1 month |
| Shipping | Room temperature in continental US; may vary elsewhere |
Tel: 0086-25-52397805
Email: info@alchemist-chem.com

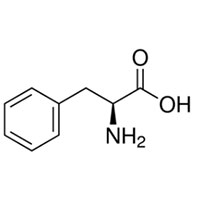
| Common Names | L-PHENYLALANINE | ||
|---|---|---|---|
| Structure |  |
||
| CAS No. | 63-91-2 | Boiling Point (℃) | 307.5±30.0 °C at 760 mmHg |
| Molecular Weight | 165.19 | Melting Point (℃) | 270-275ºC (dec.)(lit.) |
| Density | 1.2±0.1 g/cm3 | Vapor Specific Gravity | N/A |
| Molecular Formula | C9H11NO2 | Flash Point (℃) | 139.8±24.6 °C |
| Solubility | N/A | Autoignition Temperature (℃) | N/A |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter | ||
|---|---|---|---|
| Hazard Codes | T+:Very toxic | ||
| Safety Phrases | S1-S28-S45 | ||
| RIDADR | NONH for all modes of transport | ||
| WGK Germany | 3 | ||
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention. |
| Description | L-Phenylalanine is an antagonist at α2δ calcium channels with a Ki of 980 nM. IC50 Value: 980 nM [1]Target: Calcium ChannelL-Phenylalanine (LPA) is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form proteins. In the brain, L-phenylalanine is a competitive antagonist at the glycine binding site of NMDA receptor and at the glutamate binding site of AMPA receptor [2, 3]. At the glycine binding site of NMDA receptor L-phenylalanine has an apparent equilibrium dissociation constant (KB) of 573 ?M estimated by Schild regression [4] which is considerably lower than brain L-phenylalanine concentration observed in untreated human phenylketonuria [5]. L-Phenylalanine also inhibits neurotransmitter release at glutamatergic synapses in hippocampus and cortex with IC50 of 980 nM, a brain concentration seen in classical phenylketonuria, whereas D-phenylalanine has a significantly smaller effect [3]. | ||
|---|---|---|---|
| Target | Km: 100-400 μM (amino acid transporter b0,+)[1] | ||
| In Vitro | DL-Lysine (Lys) is a high affinity, basic amino acid substrate for amino acid transporter b0,+ with Km value ranging from 100-400 μM[1]. | ||
| References | [1]. Mortell, K.H., et al., Structure-activity relationships of alpha-amino acid ligands for
the alpha2delta subunit of voltage-gated calcium channels. Bioorg Med Chem Lett, 2006.
16(5): p. 1138-41. [2]. Glushakov, A.V., et al., Specific inhibition of N-methyl-D-aspartate receptor function in rat hippocampal neurons by L-phenylalanine at concentrations observed during phenylketonuria. Mol Psychiatry, 2002. 7(4): p. 359-67. [3]. Glushakov, A.V., et al., L-phenylalanine selectively depresses currents at glutamatergic excitatory synapses. J Neurosci Res, 2003. 72(1): p. 116-24. [4]. Glushakov, A.V., et al., Long-term changes in glutamatergic synaptic transmission in phenylketonuria. Brain, 2005. 128(Pt 2): p. 300-7. [5]. Moller, H.E., et al., Brain imaging and proton magnetic resonance spectroscopy in patients with phenylketonuria. Pediatrics, 2003. 112(6 Pt 2): p. 1580-3. |
||