
| N/A |
| Packing | |
|---|---|
| Storage | Powder -20°C 3 years 4°C 2 years;In solvent -80°C 6 months, -20°C 1 month |
| Shipping | Room temperature in continental US; may vary elsewhere |
Tel: 0086-25-52397805
Email: info@alchemist-chem.com

| Common Names | L-Histidine | ||
|---|---|---|---|
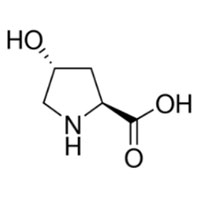
| Structure |  |
||
| CAS No. | 51-35-4 | Boiling Point (℃) | 355.2±42.0 °C at 760 mmHg |
| Molecular Weight | 131.13 | Melting Point (℃) | 273 °C (dec.)(lit.) |
| Density | 1.4±0.1 g/cm3 | Vapor Specific Gravity | N/A |
| Molecular Formula | C5H9NO3 | Flash Point (℃) | 168.6±27.9 °C |
| Solubility | 357.8 g/L (20 º C) | Autoignition Temperature (℃) | N/A |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter | ||
|---|---|---|---|
| Hazard Codes | T+:Very toxic | ||
| Safety Phrases | S1-S28-S45 | ||
| RIDADR | NONH for all modes of transport | ||
| WGK Germany | 3 | ||
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention. |
| Description | L-Hydroxyproline, one of the hydroxyproline (Hyp) isomers, is a useful chiral building block in the production of many pharmaceuticals. | ||
|---|---|---|---|
| Target | Human Endogenous Metabolite | ||
| In Vitro | L-Hydroxyproline (Trans-4-hydroxy-L-proline;Trans-Hyp) has been widely used in medicine, biochemistry, food, cosmetic and other aspects of industry. Additionally, L-Hydroxyproline has also been found in the composition of some secondary metabolites such as actinomycins and echinocandins. L-Hydroxyproline is manufactured industrially most by acid hydrolysis of mammalian collagen because of its rich amount in the collagen[1]. | ||
| References | [1]. Yi Y, et al. Biosynthesis of trans-4-hydroxyproline by recombinant strains of Corynebacterium glutamicum and Escherichia coli. BMC Biotechnol. 2014 May 19;14:44. | ||