
| N/A |
| Packing | |
|---|---|
| Storage | Powder -20°C 3 years 4°C 2 years;In solvent -80°C 6 months, -20°C 1 month |
| Shipping | Room temperature in continental US; may vary elsewhere |
Tel: 0086-25-52397805
Email: sales7@alchemist-chem.com

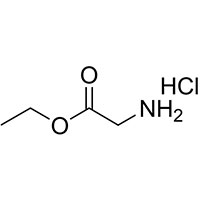
| Common Names | Glycine ethyl ester hydrochloride | ||
|---|---|---|---|
| Structure |  |
||
| CAS No. | 623-33-6 | Boiling Point (℃) | 109.5 |