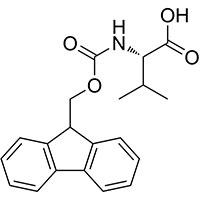
Synonyms
L-Valine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-
N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-valine
N-Fmoc-Val-OH (N-Fmoc-L-valine)
FMco-Val-OH
N-FMOC-L-VAL
FMOC-L-VAL-OH
FMOC-VAL
FMOC-VAL-OH
MFCD00037124
N-((9H-Fluoren-9-ylmethoxy)carbonyl)-L-valine
FMOC-VALINE
FMOC-L-VALINE
N-Fmoc-L-valine
FMOC-L-VAL
N-FMOC-VAL-OH
Fmoc-Valine-OH
EINECS 272-515-0
Product Description
Fmoc-L-Valine is a cutting-edge chemical compound that offers superior protection for amino acids during
various chemical synthesis processes. With its exceptional properties and wide-ranging applications in
the field of chemical engineering, Fmoc-L-Valine has gained significant recognition in the global
market. This product introduction aims to highlight the key features, benefits, and potential
applications of Fmoc-L-Valine, emphasizing its crucial role in safeguarding amino acids and facilitating
efficient synthesis in the ever-evolving chemical industry.
Advanced Amino Acid Protection:
Fmoc-L-Valine provides advanced protection for amino acids, ensuring their stability and preventing
undesired reactions during chemical synthesis. With its innovative protective group, Fmoc-L-Valine
effectively shields the amino acid moiety, preserving the reactivity of functional groups and minimizing
side reactions. This precise protection allows for efficient and high-yielding synthesis, leading to the
production of high-quality amino acid derivatives.
Versatile Applications:
Fmoc-L-Valine finds extensive use in a wide range of chemical synthesis applications. Its superior
protection capabilities make it an indispensable tool in peptide synthesis, where it plays a vital role
in maintaining the integrity and reactivity of amino acids during the formation of peptide bonds.
Additionally, Fmoc-L-Valine is valuable in the synthesis of pharmaceutical intermediates, agrochemicals,
and specialty chemicals, enabling the efficient production of diverse compounds in the pharmaceutical
and chemical industries.
Enhanced Chemical Compatibility:
Fmoc-L-Valine demonstrates excellent compatibility with various solvents, reagents, and reaction
conditions commonly employed in chemical synthesis. This versatility allows for seamless integration
into different synthetic methodologies, making Fmoc-L-Valine suitable for a wide range of applications.
Its compatibility and reliability contribute to improved synthetic efficiency and consistent product
quality.
Global Market Impact:
Fmoc-L-Valine has made a significant impact on the global chemical market, establishing itself as a
leading solution for amino acid protection. Its exceptional performance, consistent quality, and
cost-effectiveness have garnered widespread recognition and trust from researchers, scientists, and
industry professionals. The increasing demand for Fmoc-L-Valine reflects its effectiveness in optimizing
synthetic processes and delivering high-quality amino acid derivatives.
Conclusion:
Fmoc-L-Valine represents a significant advancement in amino acid protection for chemical synthesis. Its
advanced properties, versatile applications, and enhanced chemical compatibility make it an essential
component in modern chemical engineering. By choosing Fmoc-L-Valine, researchers and manufacturers can
confidently protect amino acids, enhance synthetic efficiency, and accelerate the development of
pharmaceuticals, agrochemicals, and specialty chemicals. Fmoc-L-Valine is a key driver of progress in
the global chemical industry, enabling efficient and reliable synthesis processes while maintaining
superior product quality.