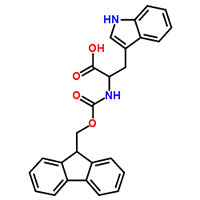
Synonyms
fmoc-D-Trp-OH
FMOC-TRP
MFCD00037126
FMOC-TRYPTOPHAN
L-TRYPTOPHAN-N-FMOC
N-Fmoc-D-Trp-OH
N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-tryptophan
Tryptophan, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-
Nalpha-FMOC-L-Trypto
Fmoc-Trp-OH
FMOC-L-TRP-OH
N-Fmoc-L-Trp-OH
N-((9H-Fluoren-9-ylmethoxy)carbonyl)-L-tryptophan
EINECS 252-706-5
N-[(9H-Fluoren-9-ylmethoxy)carbonyl]tryptophan
Nα-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-tryptophan
FMOC-L-TRP
N-Fmoc-D-tryptophan
Fmoc-L-tryptophan
Product Description
Fmoc-L-Tryptophan is a cutting-edge chemical compound specifically designed to provide superior
protection for amino acids during various chemical processes. With its exceptional properties and
versatile applications in the field of chemical synthesis, Fmoc-L-Tryptophan has gained significant
recognition in the global market. This product introduction aims to highlight the key features,
benefits, and potential applications of Fmoc-L-Tryptophan, emphasizing its crucial role in safeguarding
amino acids and enabling efficient synthesis in the ever-evolving chemical industry.
Advancements in Chemical Engineering:
Fmoc-L-Tryptophan represents a significant advancement in chemical engineering, offering enhanced
protection and stability for amino acids during various synthetic procedures. Developed through
cutting-edge research and innovative manufacturing techniques, this compound ensures the integrity of
amino acids and prevents unwanted reactions, allowing for efficient and high-yielding chemical
transformations.
Superior Amino Acid Protection:
Fmoc-L-Tryptophan provides superior protection for amino acids by forming a robust and reversible bond.
This protective group effectively shields the amino acid moiety, preserving the reactivity of functional
groups and preventing unwanted side reactions. The precise control over chemical transformations
facilitated by Fmoc-L-Tryptophan leads to improved synthetic efficiency, resulting in the production of
high-quality amino acid derivatives with excellent yield and purity.
Wide Range of Applications:
Fmoc-L-Tryptophan finds extensive utility in various chemical synthesis applications due to its
exceptional protective properties. It is particularly valuable in peptide synthesis, pharmaceutical
manufacturing, and the preparation of complex organic molecules. Fmoc-L-Tryptophan demonstrates
compatibility with a wide range of solvents and reagents, making it an ideal choice for both traditional
and advanced synthetic methodologies.
Global Market Impact:
Fmoc-L-Tryptophan has made a significant impact on the global chemical market, establishing itself as a
leading solution for amino acid protection. Its consistent quality, reliability, and cost-effectiveness
have garnered a strong reputation among researchers, scientists, and industry professionals. The product
has witnessed a substantial increase in demand, attracting a growing customer base across different
regions. Fmoc-L-Tryptophan has become an essential tool for chemical manufacturers aiming to optimize
their synthetic processes and deliver high-quality amino acid derivatives.
Conclusion:
Fmoc-L-Tryptophan revolutionizes amino acid protection in chemical synthesis, offering enhanced
stability, compatibility, and reliability. Its broad applications and positive impact on the global
market position it as a key component of modern chemical engineering. By choosing Fmoc-L-Tryptophan,
researchers and manufacturers can confidently protect amino acids, enhance synthetic efficiency, and
accelerate the development of pharmaceuticals, specialty chemicals, and other complex organic
compounds.