Synonyms
MFCD00065670
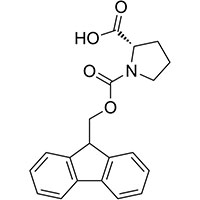
Fmoc-L-Pro-OH
EINECS 276-259-0
Fmoc-L-proline
1-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-proline
Fmoc-L-proline-OH
1,2-Pyrrolidinedicarboxylic acid, 1-(9H-fluoren-9-ylmethyl) ester, (2S)-
Fmoc-Pro-OH
Product Description
Fmoc-L-Proline is a cutting-edge product that combines amino acid protection with the field of chemical
synthesis. With its unique features and wide range of applications, Fmoc-L-Proline has gained
significant traction in the international market. This product introduction aims to highlight the key
attributes, benefits, and potential applications of Fmoc-L-Proline, underscoring its pivotal role in
safeguarding amino acids and promoting efficient synthesis in the dynamic chemical industry.
Optimal Amino Acid Protection:
Fmoc-L-Proline offers optimal protection for amino acids, ensuring their stability and preventing
unwanted reactions during chemical synthesis. Its advanced protective group shields the amino acid,
preserving the reactivity of functional groups and minimizing side reactions. This precise protection
enables the synthesis of complex molecules and facilitates the production of high-quality amino acid
derivatives.
Versatile Applications:
Fmoc-L-Proline finds versatile applications in various chemical synthesis processes. Its exceptional
protection capabilities make it a valuable component in peptide synthesis, safeguarding the amino acid
backbone during peptide bond formation. Fmoc-L-Proline is also widely used in the synthesis of
pharmaceuticals, agrochemicals, and specialty chemicals, enabling efficient production of diverse
compounds in the global chemical industry.
Enhanced Solubility and Stability:
Fmoc-L-Proline exhibits enhanced solubility and stability, making it compatible with a wide range of
solvents, reagents, and reaction conditions. Its improved solubility and stability properties allow for
seamless integration into different chemical processes, facilitating efficient and reliable synthesis.
By utilizing Fmoc-L-Proline, researchers can enhance reaction efficiency, minimize undesired side
reactions, and produce high-quality amino acid derivatives.
Global Market Impact:
Fmoc-L-Proline has made a significant impact on the global chemical market, emerging as a leading
solution for amino acid protection. Its exceptional performance, reliable quality, and
cost-effectiveness have garnered recognition and trust from researchers, scientists, and industry
professionals worldwide. The growing demand for Fmoc-L-Proline reflects its effectiveness in optimizing
synthetic processes and delivering high-quality amino acid derivatives.
Conclusion:
Fmoc-L-Proline represents a significant advancement in amino acid protection for chemical synthesis. Its
optimal protection capabilities, versatile applications, and enhanced solubility and stability make it a
valuable tool in modern chemical engineering. By harnessing Fmoc-L-Proline, researchers and
manufacturers can confidently protect amino acids, enhance synthetic efficiency, and accelerate the
development of pharmaceuticals, agrochemicals, and specialty chemicals. Fmoc-L-Proline is driving
progress in the global chemical industry, enabling efficient and reliable synthesis processes while
ensuring superior product quality. Choose Fmoc-L-Proline for unrivaled amino acid protection and unlock
new possibilities in chemical synthesis.