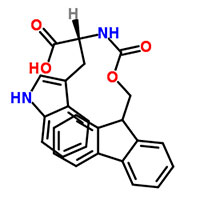
Synonyms
FMOC-D-TRP
N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-D-tryptophan
Na-(9-Fluorenylmethoxycarbonyl)-D-tryptophan
D-Tryptophan, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-
Fmoc-D-Trp-OH
N-Fmoc-D-tryptophan
Nalpha-(9-Fluorenylmethoxycarbonyl)-D-tryptophan
MFCD00062954
Fmoc-D-tryptophan
N-Fmoc-D-Trp-OH
Product Description
Fmoc-D-Tryptophan is an innovative compound that plays a vital role in protecting amino acids during
various chemical synthesis processes. With its exceptional properties and versatile applications in the
field of chemical engineering, Fmoc-D-Tryptophan has gained significant recognition in the global
market. This product introduction aims to showcase the key features, advantages, and potential
applications of Fmoc-D-Tryptophan, emphasizing its crucial role in safeguarding amino acids and
facilitating efficient synthesis in the ever-evolving chemical industry.
Optimal Amino Acid Protection:
Fmoc-D-Tryptophan offers optimal protection for amino acids, ensuring their stability and preventing
unwanted reactions during chemical synthesis. With its advanced protective group, Fmoc-D-Tryptophan
effectively shields the amino acid moiety, preserving the reactivity of functional groups and minimizing
side reactions. This precise protection enables the efficient synthesis of complex molecules and
facilitates the production of high-quality amino acid derivatives.
Versatile Applications:
Fmoc-D-Tryptophan finds extensive applications in a wide range of chemical synthesis processes. Its
exceptional protection capabilities make it an indispensable tool in peptide synthesis, where it plays a
critical role in preserving the integrity and reactivity of amino acids during peptide bond formation.
Fmoc-D-Tryptophan is also widely used in the synthesis of pharmaceuticals, natural products, and
bioactive compounds, enabling the efficient production of diverse molecules in the pharmaceutical and
chemical industries.
Improved Solubility and Stability:
Fmoc-D-Tryptophan exhibits improved solubility and stability, making it highly compatible with various
solvents, reagents, and reaction conditions. Its enhanced solubility and stability properties facilitate
seamless integration into different chemical processes, ensuring efficient and reliable synthesis. The
use of Fmoc-D-Tryptophan enhances reaction efficiency, minimizes unwanted side reactions, and
contributes to the production of high-quality amino acid derivatives.
Global Market Impact:
Fmoc-D-Tryptophan has made a significant impact on the global chemical market, establishing itself as a
leading solution for amino acid protection. Its exceptional performance, reliable quality, and
cost-effectiveness have gained widespread recognition and trust from researchers, scientists, and
industry professionals. The growing demand for Fmoc-D-Tryptophan reflects its effectiveness in
optimizing synthetic processes and delivering high-quality amino acid derivatives.
Conclusion:
Fmoc-D-Tryptophan represents a groundbreaking advancement in amino acid protection for chemical
synthesis. Its optimal protection capabilities, versatile applications, and improved solubility and
stability make it an invaluable asset in modern chemical engineering. By choosing Fmoc-D-Tryptophan,
researchers and manufacturers can confidently protect amino acids, enhance synthetic efficiency, and
accelerate the development of pharmaceuticals, natural products, and bioactive compounds.
Fmoc-D-Tryptophan is driving progress in the global chemical industry, enabling efficient and reliable
synthesis processes while ensuring superior product quality.