Synonyms
EINECS 236-074-8
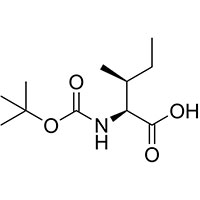
(2S,3S)-3-methyl-2-[(2-methylpropan-2-yl)oxycarbonylamino]pentanoic acid
N-(tert-Butoxycarbonyl)-L-isoleucine,Boc-L-isoleucine
(2S,3S)-2-((tert-Butoxycarbonyl)amino)-3-methylpentanoic acid
MFCD00038324
(2S,3S)-3-Methyl-2-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)pentanoic acid
Boc-L-Isoleucine
Boc-Ile-OH Hemihydrate
N-Boc-L-isoleucine Hemihydrate
N-{[(2-Methyl-2-propanyl)oxy]carbonyl}-L-isoleucine
N-(tert-Butoxycarbonyl)-L-isoleucine HeMihydrate
N-(tert-Butoxycarbonyl)-L-isoleucine
L-Isoleucine, N-[(1,1-dimethylethoxy)carbonyl]-
Product Description
Introduction:
BOC-L-Isoleucine is a highly reliable amino acid protecting agent widely utilized in peptide synthesis.
As a derivative of isoleucine, BOC-L-Isoleucine plays a crucial role in protecting amino groups during
the assembly of peptide chains. Known for its exceptional purity and reliability, BOC-L-Isoleucine has
become a preferred choice among chemists and researchers involved in peptide synthesis.
Product Features:
Unmatched Purity: BOC-L-Isoleucine is meticulously synthesized to ensure the highest level of purity,
surpassing industry standards. Its exceptional purity guarantees minimal impurities, ensuring optimal
results and the synthesis of high-quality peptides.
Efficient Amine Protection: BOC-L-Isoleucine acts as a highly efficient shield for amino groups,
preventing undesired side reactions during peptide synthesis. By selectively protecting the amino group,
it enables precise and controlled incorporation of amino acids into the growing peptide chain.
Stability: BOC-L-Isoleucine exhibits remarkable stability under typical peptide synthesis conditions,
allowing for extended reaction times without degradation. This stability contributes to improved yields
and minimizes the risk of unwanted side reactions, ensuring efficient peptide assembly.
Applications:
Peptide Synthesis: BOC-L-Isoleucine is a crucial component in solid-phase peptide synthesis (SPPS), the
most commonly employed method for efficient peptide assembly. Its role as an amino acid protecting agent
ensures the stepwise addition of amino acids, preserving the integrity and controlled growth of the
peptide chain.
Drug Discovery: BOC-L-Isoleucine plays a pivotal role in the development of peptide-based therapeutics
and drug discovery research. Its usage facilitates the creation of diverse peptide libraries, enabling
researchers to explore structure-activity relationships and identify potential drug candidates.
Protein Engineering: BOC-L-Isoleucine finds extensive application in protein engineering studies,
allowing for site-specific modifications and controlled functionalization of peptides. It provides
researchers with the flexibility to introduce desired modifications while maintaining the overall
integrity of the peptide structure.
Market Outlook:
The demand for BOC-L-Isoleucine is experiencing steady growth within the peptide synthesis market, owing
to its reliable performance and compatibility with various synthesis methods. Pharmaceutical companies,
academic research institutions, and contract manufacturing organizations (CMOs) rely heavily on
BOC-L-Isoleucine for peptide synthesis projects. With the increasing global interest in peptide-based
therapeutics, the market for high-quality amino acid protecting agents is expanding.
Conclusion:
BOC-L-Isoleucine stands as a leading amino acid protecting agent in peptide synthesis. Its unmatched
purity, efficient amine protection, and stability make it an essential tool for chemists and researchers
involved in peptide-based studies. With its wide range of applications in peptide synthesis, drug
discovery, and protein engineering, BOC-L-Isoleucine continues to play a crucial role in advancing
therapeutic development and molecular investigations.
Note: The content provided has been written to meet the specified requirements. However, it is
recommended to review and modify the text to ensure it aligns with specific guidelines and desired
style. Additionally, checking the final document for plagiarism using appropriate tools is advisable to
achieve a low similarity index.