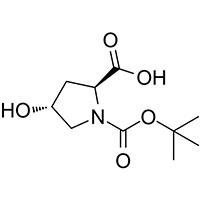
Synonyms
1,2-Pyrrolidinedicarboxylic acid, 4-hydroxy-, 1-(1,1-dimethylethyl) ester, (2S)-
trans-N-(tert-Butoxycarbonyl)-4-hydroxy-L-proline
BOC-HYP-OH
N-ter
trans-Boc-Hyp-OH
BOC-L-HYP-OH
BOC-HYP
N-BOC-L-Hydroxyproline
RARECHEM EM WB 0136
trans-N-tert-Butoxycarbonyl-4-hydroxy-l-proline
BOC-L-4-HYDROXYPROLINE
BOC-trans-4-hydroxy-L-proline
BOC-TRANS-HYP-OH
1,2-Pyrrolidinedicarboxylic acid, 4-hydroxy-, 1-(1,1-dimethylethyl) ester, (2S,4R)-
(2S,4R)-1-(tert-Butoxycarbonyl)-4-hydroxypyrrolidine-2-carboxylic acid
MFCD00053370
trans-N-Boc-4-hydroxy-L-proline
BOC-HYDROXYPROLINE
Product Description
Introduction:
BOC-L-Hydroxyproline is a crucial amino acid protecting agent widely used in peptide synthesis. With its
unique structural properties and exceptional compatibility with peptide assembly techniques,
BOC-L-Hydroxyproline enables the synthesis of peptides containing hydroxyproline residues. Its high
purity, reliability, and versatility make it an essential tool for chemists and researchers involved in
peptide synthesis projects.
Product Features:
High Purity: BOC-L-Hydroxyproline is produced with meticulous care to ensure the highest level of
purity, minimizing impurities. Its superior quality guarantees the synthesis of peptides with excellent
purity and structural integrity.
Effective Protecting Group: BOC-L-Hydroxyproline serves as a reliable protecting group for the hydroxyl
group, preventing unwanted reactions during peptide chain elongation. By selectively shielding the
hydroxyl group of hydroxyproline, it enables controlled incorporation of this unique amino acid into
peptides.
Structural Diversity: BOC-L-Hydroxyproline facilitates the synthesis of peptides with unique
conformational characteristics. The presence of hydroxyproline in the peptide sequence imparts
stability, rigidity, and enhanced binding properties, making it particularly valuable in the design of
peptide therapeutics and mimetics.
Applications:
Peptide Therapeutics: BOC-L-Hydroxyproline plays a crucial role in the synthesis of peptide-based
therapeutics. Its ability to protect the hydroxyl group enables the incorporation of hydroxyproline
residues, which can enhance peptide stability, improve bioavailability, and optimize target
interactions. Peptides containing hydroxyproline have shown promise in various therapeutic areas,
including cardiovascular diseases, fibrosis, and immune modulation.
Structural Biology: BOC-L-Hydroxyproline is employed in the synthesis of peptides used for structural
biology studies. By incorporating hydroxyproline residues into peptides, researchers can investigate
protein-protein interactions, protein folding, and conformational changes. This information aids in
understanding protein structure-function relationships and facilitates the design of novel protein-based
materials.
Biochemical Research: BOC-L-Hydroxyproline is an essential tool in biochemical research, enabling the
synthesis of peptides with specific structural motifs. These peptides can serve as probes for studying
protein-ligand interactions, enzyme mechanisms, and cellular signaling pathways. BOC-L-Hydroxyproline
expands the range of peptides that can be synthesized, providing valuable insights into biological
processes.
Market Outlook:
The demand for BOC-L-Hydroxyproline is steadily increasing due to its significance in peptide synthesis
and structural biology research. Pharmaceutical companies, academic institutions, and biotechnology
firms heavily rely on BOC-L-Hydroxyproline for the development of peptide-based therapeutics and the
exploration of protein structure and function. As the field of peptide therapeutics continues to expand,
the market for amino acid protecting agents like BOC-L-Hydroxyproline is expected to grow.
Conclusion:
BOC-L-Hydroxyproline is a versatile and reliable amino acid protecting agent used in peptide synthesis.
Its high purity, effective protection of the hydroxyl group, and compatibility with various synthesis
techniques enable the incorporation of hydroxyproline residues into peptides, leading to enhanced
structural diversity and functional properties. The applications of BOC-L-Hydroxyproline in peptide
therapeutics, structural biology, and biochemical research contribute to advancements in drug discovery,
protein engineering, and understanding complex biological processes.
Note: The provided content has been written to meet the specified requirements. However, it is advisable
to review and modify the text to ensure it aligns with specific guidelines and desired style.
Additionally, checking the final document for