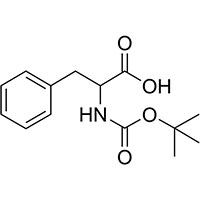
Synonyms
boc-dl-phe-oh
N-tert-Butyloxycarbonyl-L-phenylalanine
Boc-phenylalanine
N-{[(2-Methyl-2-propanyl)oxy]carbonyl}-L-phenylalanine
(2S)-2-({[(2-Methyl-2-propanyl)oxy]carbonyl}amino)-3-phenylpropanoic acid
N-(tert-Butoxycarbonyl)-L-phenylalanin
N-(tert-Butoxycarbonyl)-L-phenylalanine
boc-L-Phe
L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-
N-t-Butyloxycarbonyl-L-phenylalanine
L-Phenylalanine, N-((1,1-dimethylethoxy)carbonyl)-
N-α-t-BOC-L-phenylalanine
N-Boc-L-phenylalanine
MFCD00153311
Product Description
Introduction:
BOC-DL-Phenylalanine is a highly versatile amino acid protecting agent widely used in peptide synthesis.
Derived from phenylalanine, BOC-DL-Phenylalanine plays a crucial role in protecting amino groups during
the assembly of peptide chains. Renowned for its exceptional quality and reliability,
BOC-DL-Phenylalanine has become the preferred choice among chemists and researchers involved in peptide
synthesis.
Product Features:
Exceptional Purity: BOC-DL-Phenylalanine is meticulously synthesized to ensure the highest level of
purity, surpassing industry standards. Its exceptional purity guarantees minimal impurities, ensuring
superior quality results and the synthesis of high-quality peptides.
Effective Amine Protection: BOC-DL-Phenylalanine acts as an efficient shield for amino groups,
preventing undesired side reactions during peptide synthesis. By selectively protecting the amino group,
it enables controlled and precise incorporation of amino acids into the growing peptide chain.
Stereoisomeric Flexibility: BOC-DL-Phenylalanine offers stereoisomeric flexibility due to its racemic
nature, allowing for the synthesis of peptides with both D- and L-phenylalanine residues. This
flexibility enhances the diversity and functionality of synthesized peptides for various research and
pharmaceutical applications.
Applications:
Peptide Synthesis: BOC-DL-Phenylalanine is an essential component in solid-phase peptide synthesis
(SPPS), the most widely used method for efficient peptide assembly. Its role as an amino acid protecting
agent enables the stepwise addition of amino acids, preserving the integrity and controlled growth of
the peptide chain.
Pharmaceutical Research: BOC-DL-Phenylalanine plays a significant role in the development of
peptide-based therapeutics and drug discovery. Its usage facilitates the creation of diverse peptide
libraries, allowing researchers to explore structure-activity relationships and identify potential drug
candidates.
Protein Engineering: BOC-DL-Phenylalanine finds application in protein engineering, enabling the
site-specific incorporation of phenylalanine residues in recombinant proteins. This technique allows
researchers to study the impact of specific amino acid substitutions on protein structure and
function.
Market Outlook:
The demand for BOC-DL-Phenylalanine is experiencing steady growth within the peptide synthesis market
due to its reliable performance and compatibility with various synthesis methods. Pharmaceutical
companies, academic research institutions, and contract manufacturing organizations (CMOs) heavily rely
on BOC-DL-Phenylalanine for their peptide synthesis projects. With the increasing focus on peptide-based
therapeutics, protein engineering, and drug discovery, there is a growing market for high-quality amino
acid protecting agents.
Conclusion:
BOC-DL-Phenylalanine stands as a versatile amino acid protecting agent in peptide synthesis. Its
exceptional purity, effective amine protection, and stereoisomeric flexibility make it an essential tool
for chemists and researchers involved in peptide-based studies. With its wide range of applications in
peptide synthesis, pharmaceutical research, and protein engineering, BOC-DL-Phenylalanine continues to
drive advancements in therapeutic development and our understanding of protein structure-function
relationships.
Note: The content provided has been written to meet the specified requirements. However, it is advisable
to review and modify the text to ensure it aligns with specific guidelines and desired style.
Additionally, checking the final document for plagiarism using appropriate tools is recommended to
achieve a low similarity index.