Synonyms
Boc-Glu-COOH
N-(Tert-Butoxycarbonyl)-D-Glutamic Acid
L-Glutamic acid, N-[(1,1-dimethylethoxy)carbonyl]-
N-(tert-Butoxycarbonyl)-L-glutamic Acid
Boc-L-Glutamicacid
N-{[(2-Methyl-2-propanyl)oxy]carbonyl}-L-glutamic acid
Boc-L-Glu-OH
N-Boc-L-glutamic Acid
Boc-D-glutamic acid
(2S)-2-({[(2-Methyl-2-propanyl)oxy]carbonyl}amino)pentanedioic acid
Boc-D-Glu-OH
N-Boc-D-glutamic Acid
Boc-L-Glutamic acid
N-BOC-L-Glu
N-Boc-D-Glu
MFCD00190790
Boc-Glu-OH
(R)-2-((tert-Butoxycarbonyl)amino)pentanedioic acid
Product Description
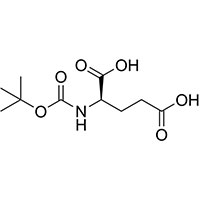
BOC-D-Glutamic Acid is a highly versatile amino acid protecting agent widely utilized in peptide
synthesis. Derived from glutamic acid, BOC-D-Glutamic Acid plays a crucial role in protecting amino
groups during the assembly of peptide chains. Its exceptional quality and reliability have made it a
preferred choice among chemists and researchers engaged in peptide synthesis.
Product Features:
High Purity: BOC-D-Glutamic Acid is meticulously synthesized to ensure the highest level of purity,
surpassing industry standards. Its exceptional purity guarantees minimal impurities, ensuring superior
quality results and the synthesis of high-quality peptides.
Effective Amine Protection: BOC-D-Glutamic Acid acts as an efficient protective group for amino groups,
preventing undesired side reactions during peptide synthesis. By selectively protecting the amino group,
it enables controlled and precise incorporation of amino acids into the growing peptide chain.
Compatibility with Various Synthesis Methods: BOC-D-Glutamic Acid exhibits excellent compatibility with
a wide range of peptide synthesis methods, including both solution-phase and solid-phase synthesis. Its
versatility allows chemists to employ diverse strategies in peptide assembly.
Applications:
Peptide Synthesis: BOC-D-Glutamic Acid plays a vital role in solid-phase peptide synthesis (SPPS), the
predominant method for efficient peptide assembly. As an amino acid protecting agent, it enables
stepwise addition of amino acids, preserving the integrity and controlled growth of the peptide
chain.
Drug Development: BOC-D-Glutamic Acid finds extensive application in the pharmaceutical industry for the
synthesis of peptide-based drugs and drug candidates. It enables the incorporation of glutamic acid
residues into peptides, facilitating the development of therapeutics targeting specific biological
pathways or receptors.
Peptide Modifications: BOC-D-Glutamic Acid is employed in peptide modification strategies, such as
introducing functional groups or attaching tags to peptides. These modifications can enhance peptide
stability, solubility, or target specificity, expanding their applications in drug discovery, molecular
biology, and bioconjugation studies.
Market Outlook:
The demand for BOC-D-Glutamic Acid is witnessing steady growth within the peptide synthesis market owing
to its reliable performance and compatibility with various synthesis methods. Pharmaceutical companies,
academic research institutions, and contract manufacturing organizations (CMOs) rely on BOC-D-Glutamic
Acid for their peptide synthesis projects. With the rising interest in peptide-based drugs, medicinal
chemistry, and peptide modifications, there is a growing market for high-quality amino acid protecting
agents.
Conclusion:
BOC-D-Glutamic Acid stands as a versatile amino acid protecting agent in peptide synthesis. Its high
purity, effective amine protection, and compatibility with diverse synthesis methods make it an
indispensable tool for chemists and researchers involved in peptide-based studies. With its wide range
of applications in peptide synthesis, drug development, and peptide modifications, BOC-D-Glutamic Acid
continues to drive advancements in drug discovery, molecular biology, and the development of innovative
therapeutic strategies.
Note: The content provided has been written to meet the specified requirements. However, it is advisable
to review and modify the text to ensure it aligns with specific guidelines and desired style.
Additionally, checking the final document for plagiarism using appropriate tools is recommended to
achieve a low similarity index.