
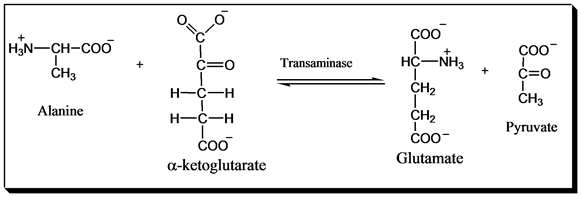
Figure 3. Alanine transamination
Transamination is a critical reaction that allows the transfer of amino groups between amino acids and α-keto
acids. The enzyme alanine transaminase (ALT) catalyzes the reversible reaction between L-alanine and
α-ketoglutarate to form pyruvate and L-glutamate[1]:
L-Alanine + α-Ketoglutarate ⇌ Pyruvate + L-Glutamate
This ping-pong reaction occurs via a pyridoxal phosphate (PLP) cofactor covalently bound to the enzyme. In the
first half-reaction, the amino group of L-alanine is transferred to the PLP cofactor, generating pyridoxamine
phosphate (PMP) and releasing pyruvate. In the second half-reaction, the amino group is transferred from PMP
to α-ketoglutarate, regenerating PLP and producing L-glutamate[2].
Transamination is crucial for shuttling nitrogen between various amino acids and for the synthesis of
nonessential amino acids. The products of alanine transamination, pyruvate and glutamate, can be further
metabolized in important pathways such as the citric acid cycle, gluconeogenesis, and the urea cycle[3].
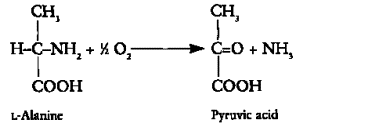
Figure 4. Oxidative deamination
Oxidative deamination removes the amino group from amino acids, releasing it as ammonia (NH3). While alanine
does not directly undergo oxidative deamination, the glutamate produced by alanine transamination can be
oxidatively deaminated by glutamate dehydrogenase[3, 4]:
L-Glutamate + NAD+ + H2O → α-Ketoglutarate + NADH + NH4+
This reaction occurs primarily in the liver and provides a means to eliminate excess nitrogen as urea. The
α-ketoglutarate formed can enter the citric acid cycle for energy production[3].
Glucose-Alanine Cycle
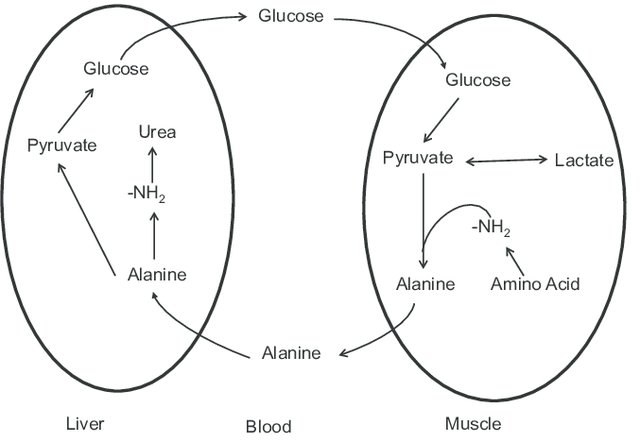
Figure 5. The glucose–alanine cycle. Alanine is synthesized in muscle by transamination
of glucose-derived pyruvate, and released into the bloodstream. In the liver, the carbon skeleton of alanine
is reconverted to glucose, and released into the bloodstream where it is available for uptake by muscle and
resynthesis of alanine[5].
The glucose-alanine cycle, also known as the Cahill cycle, is a metabolic pathway that allows for the
transport of amino groups from skeletal muscle to the liver[6, 7]. In muscle, amino acids are catabolized for
energy, with the resulting amino groups transferred to pyruvate via transamination to form alanine. Alanine
then enters the bloodstream and is taken up by the liver, where it is converted back to pyruvate and glucose
via gluconeogenesis. The newly synthesized glucose returns to the muscle to be used for energy production.
This cycle effectively shuttles amino groups to the liver for urea synthesis while providing a renewable
source of glucose for muscle energy needs. It is particularly important during prolonged fasting or exercise
when glucose levels are low and amino acid catabolism increases[2, 6].
D-Alanine, the enantiomer of L-alanine, is a key component of bacterial cell wall
peptidoglycan. The enzyme alanine racemase converts L-alanine to D-alanine, which is then incorporated into
the peptidoglycan structure via a series of enzymatic reactions [4, 8-10].
D-Alanine first condenses with D-glutamate to form a dipeptide, which is then added to the UDP-MurNAc
tripeptide precursor. The resulting UDP-MurNAc-pentapeptide is subsequently linked to the growing
peptidoglycan chain. Cross-linking between peptidoglycan strands occurs through the D-alanine residues,
providing structural rigidity to the cell wall [4, 8, 9].
Inhibition of D-alanine synthesis or incorporation into peptidoglycan is a common target for antibiotics, as
it disrupts cell wall integrity and leads to bacterial cell lysis [8, 10].
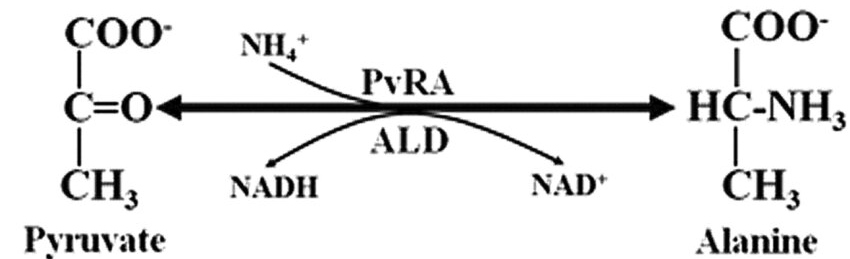
Figure 6. alanine dehydrogenase reaction
Some microorganisms express alanine dehydrogenase (AlaDH), an enzyme that catalyzes the reversible reductive
amination of pyruvate to L-alanine using NADH as a cofactor [11, 12]:
Pyruvate + NH3 + NADH + H+ ⇌ L-Alanine + NAD+ + H2O
This reaction provides an alternative route for L-alanine synthesis, particularly in organisms that lack
glutamate-pyruvate transaminase. AlaDH has been purified and characterized from various bacteria and
cyanobacteria, with studies revealing diversity in subunit structure, substrate specificity, and kinetic
properties [11, 12].
The AlaDH reaction is of biotechnological interest for the production of L-alanine from glucose and ammonia,
as it allows for a more direct synthesis route compared to the traditional transaminase-based process[11].
Alanine residues in proteins can undergo oxidative modifications mediated by reactive oxygen
species (ROS) such as hydroxyl radicals (•OH). These reactions typically involve hydrogen abstraction
from the α-carbon, forming a carbon-centered radical that can further react with oxygen to generate peroxyl
radicals and hydroperoxides[13].
Oxidative damage to alanine residues can lead to protein fragmentation, cross-linking, and functional
alterations. Such modifications have been implicated in various pathological conditions associated with
oxidative stress, including aging, neurodegenerative diseases, and cardiovascular disorders[13].
In addition to direct oxidation, alanine can also participate in Maillard reactions with reducing sugars,
leading to the formation of advanced glycation end products (AGEs). These non-enzymatic glycation reactions
are known to contribute to protein damage and inflammation in diabetes and other chronic diseases.
1. Vroon, D.H. and Z. Israili, Aminotransferases. 2011.
2. Cole, A.S. and J.E. Eastoe, Biochemistry and oral biology. 2014: Butterworth-Heinemann.
3. Felkner, W. Nutrition Flexbook. Available from:
https://courses.lumenlearning.com/suny-nutrition/.
4. Garde, S., P.K. Chodisetti, and M. Reddy, Peptidoglycan: Structure, Synthesis, and
Regulation. EcoSal Plus, 2021. 9(2).
5. Ishikura, K., S.-G. Ra, and H. Ohmori, Exercise-induced changes in amino acid levels in skeletal
muscle and plasma. The Journal of Physical Fitness and Sports Medicine, 2013. 2: p.
301-310.
6. Felig, P., et al., Alanine: Key Role in Gluconeogenesis. Science, 1970.
167(3920): p. 1003-1004.
7. Information, N.C.f.B. PubChem Pathway Summary for Pathway SMP0087221, Glucose-Alanine Cycle.
2024; Available from: https://pubchem.ncbi.nlm.nih.gov/pathway/PathBank:SMP0087221.
8. Barreteau, H., et al., Cytoplasmic steps of peptidoglycan biosynthesis. FEMS microbiology
reviews, 2008. 32(2): p. 168-207.
9. Plapp, R. and J.L. Strominger, Biosynthesis of the peptidoglycan of bacterial cell walls: XVII.
Biosynthesis of peptidoglycan and of interpeptide bridges in Lactobacillus viridescens. Journal of
Biological Chemistry, 1970. 245(14): p. 3667-3674.
10. Parker, M.F.L., et al., Sensing Living Bacteria in Vivo Using d-Alanine-Derived (11)C
Radiotracers. ACS Cent Sci, 2020. 6(2): p. 155-165.
11. Gu, P., et al., Alanine dehydrogenases from four different microorganisms: characterization and
their application in L-alanine production. Biotechnology for Biofuels and Bioproducts, 2023.
16(1): p. 123.
12. Sawa, Y., et al., Purification and characterization of alanine dehydrogenase from a cyanobacterium,
Phormidium lapideum. J Biochem, 1994. 116(5): p. 995-1000.
13. Chen, H.Y., et al., Oxygen radical-mediated oxidation reactions of an alanine peptide motif -
density functional theory and transition state theory study. Chem Cent J, 2012. 6(1):
p. 33.